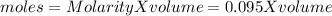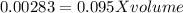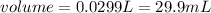Answer:
mL of NaOH required =29.9mL
Step-by-step explanation:
Let us calculate the moles of vitamin C in the tablet:
The molar mass of Vitamin C is 176.14 g/mole
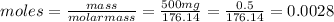
Thus we need same number of moles of NaOH to reach the equivalence point.
For NaOH solution: