Answer : The correct option is, (D) Double Replacement
Explanation :
Double-displacement reaction : A reaction in which the cation of two reactants molecule exchange their places to give two different products.
Single replacement reaction : A reaction in which the the more reactive element replace the less reactive element.
Decomposition reaction : A reaction in which the the larger molecule decomposes to give two or more smaller molecules.
Synthesis reaction : A chemical reaction where multiple substances or reactants combine to form a single product.
The given chemical reaction is:
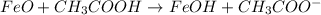
The given reaction is a double-displacement reaction in which the cation of two reactants molecule exchange their places to give two different products.
Hence, the correct option is, (D) Double Replacement