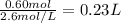Answer:
0.23 L
Step-by-step explanation:
Let's consider the following balanced equation.
3 Pb(NO₃)₂(aq) + 2 Na₃PO₄(aq) ⇄ Pb₃(PO₄)₂(s) + 6 NaNO₃(aq)
The moles of Pb(NO₃)₂ are:
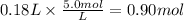
The molar ratio of Pb(NO₃)₂ to Na₃PO₄ is 3:2. The moles of Na₃PO₄ are:
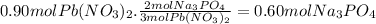
The volume of Na₃PO₄ required is: