Answer:
The correct mechanism for the reaction based on the given rate is:
Step 1 :
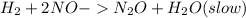
Step 2:
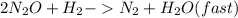
Step-by-step explanation:
The reaction rate of a muti-step reaction is determined by the slowest step of the reaction. Because of this reason, it is known as the rate-determining step of the reaction.
![rate = k[H_(2)][NO]](https://img.qammunity.org/2020/formulas/chemistry/college/ew840qvg0vs8irh2z2wjg9yy3kpvvjamie.png)
The given rate expression has the concentrations of
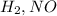 .
.
This means that the slowest step of the mechanism must involve
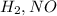 .
.
Among the proposed mechanisms, this criterion is met in the mechanism:
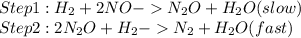
( Reason: In the above mechanism the slowest step involves
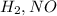 . )
. )