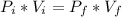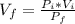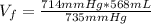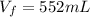Answer:
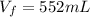
Step-by-step explanation:
Initially:
Total pressure: 735 mmHg
Water vapor pressure: 21 mmHg
Hydrogen pressure: 714 mmHg
(This is because the total pressure is divided between both gases)
When water vapor is removed:
Total pressure: 735 mmHg
Hydrogen pressure: 735 mmHg
Assuming ideal gases:
Boyle-Mariotte's Law: