Answer:
0.89 g
Step-by-step explanation:
We are given;
- 4.82 mmol of Diphenylmethanol
We are required to convert moles to mass in grams
- We need to know the relationship between mass, moles and molar mass
- Therefore, when we have molar mass and moles of a compound;
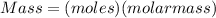
In this case;
- We need to know the molar mass of diphenylmethanol
- Molar mass of C₁₃H₁₂O = 184.234 g/mol
But, 1 mol = 1000 mmol
Thus, 4.82 mmol = 0.00482 moles
Mass of C₁₃H₁₂O = 0.00482 moles × 184.234 g/mol
= 0.888 g
= 0.89 g
Thus, the mass of C₁₃H₁₂O in 4.82 mmol is 0.89 g