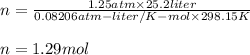Answer:
Step-by-step explanation:
This is a direct application of the equation for ideal gases.
Where:
- P = pressure = 1.25 atm
- V = volume = 25.2 liter
- R = Universal constant of gases = 0.08206 atm-liter/K-mol
- T = absolute temperature = 25.0ºC = 25 + 273.15 K = 298.15 K
- n = number of moles
Solving for n:
Substituting: