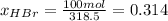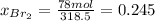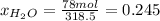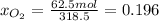Answer:
The mole fractions:
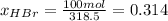
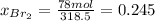
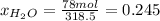
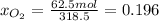
Step-by-step explanation:
The reaction described is:
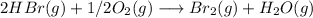
The limiting reactant is the HBr (oxygen is in excess).
a) The mass (in moles) balance for this sistem:
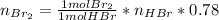
(the 0.78 is because of the fractional conversion)
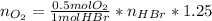
(the 1.25 is because of the oxygen excess)
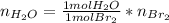
There is only one degree of freedom in this sistem, you can either deffine the moles of HBr you have or the moles of Br2 you want to produce. The other variables are all linked by the equations above.
b) Base of calculation 100 mol of HBr:

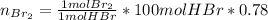
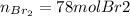
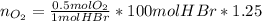
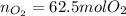
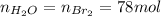
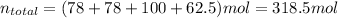
The mole fractions: