Answer:
ΔH = -44,13 kJ/mol
Step-by-step explanation:
For the following reaction:
NaOH(s) → Na⁺(aq) + OH⁻(aq)
It is possible to obtain the heat produced using:
Q = 4,18J/g°C×mass×ΔT
Where the mass is 35,1g + 199g = 234,1g
And ΔT is FinalT - InitialT → 62,6°C - 23,0°C = 39,6°C
Replacing:
Q = 38750 kJ = 38,75kJ
The enthalpy change is obtained using:
ΔH = -Q/moles of NaOH
Moles of NaOH are:
35,1g×
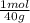 = 0,878 moles
= 0,878 moles
Thus:
ΔH = -38,75 kJ/0,878mol = -44,13 kJ/mol
I hope it helps!