Answer: 0.024 M
Step-by-step explanation:
According to the neutralization law,

where,
 = molarity of stock solution = 0.12 M
= molarity of stock solution = 0.12 M
 = volume of stock solution = 20.0 ml
= volume of stock solution = 20.0 ml
 = molarity of diluted solution = ? M
= molarity of diluted solution = ? M
 = volume of diluted solution = 100.0 ml
= volume of diluted solution = 100.0 ml
Putting in the values we get:
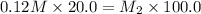
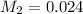
Therefore, the concentration of the resulting solution is 0.024 M.