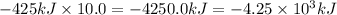Answer:
Enthalpy change when 10.0 moles of aluminium reacts with stoichiometrically equivalent ferric oxide is
 .
.
Step-by-step explanation:
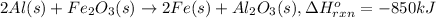
Energy change of the reaction when 2 mole of aluminium reacts with 1 mol of ferric oxide :
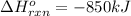
Energy change of the reaction when 1 mole of aluminium reacts :
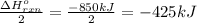
Enthalpy change when 10.0 moles of aluminium reacts with stoichiometrically equivalent ferric oxide: