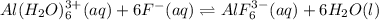Step-by-step explanation:
The potassium fluoride will dissociate into potassium ions and fluoride ions in their aqueous solution.
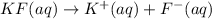
So, when 1 mol of hexaaqua aluminium (III) reacts with 6 moles of fluoride ion it gives 1 mole of hexafluoroaluminate(III).
The reaction is given as: