Answer:Frequency of the light illuminating the metal.
Step-by-step explanation:
option A:
The photons are light particles.Their speed is always constant.So,their speed does not affect the energy of the ejected electron.
option B:
The energy of the photon is given by
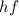 where
where
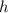 is Plancks constant and
is Plancks constant and
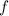 is frequency.
is frequency.
The energy of the photon converts to the energy of the emitted electron.
So,when the frequency of the photon is higher,the energy of the emitted electron increases.
option C:
The intensity of light does not have any effect on energy of the electron emitted.The intensity only determines the number of photo electrons emitted.
option D:
The charge of the electron is a constant always.So,their charge does not affect the energy of the ejected electron.