Answer: 18 grams
Step-by-step explanation:
Elevation in boiling point:
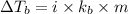
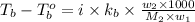
where,
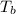 = boiling point of solution =
= boiling point of solution =
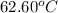
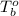 = boiling point of chloroform =
= boiling point of chloroform =
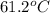
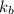 = boiling point constant of chloroform =
= boiling point constant of chloroform =
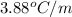
m = molality
i = Van't Hoff factor = 1 (for non-electrolyte)
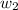 = mass of solute (hexachlorophene ) = x g
= mass of solute (hexachlorophene ) = x g
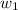 = mass of solvent (chloroform) = 125 g
= mass of solvent (chloroform) = 125 g
 = molar mass of solute (hexachlorophene ) =406.9
= molar mass of solute (hexachlorophene ) =406.9
Now put all the given values in the above formula, we get:
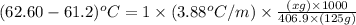
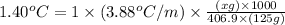
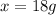
Therefore, the mass of hexachlorophene that must be added is 18 grams