To solve this problem it is necessary to apply the conservation equations of Energy.
By definition we know that the energy that is lost by water is totally equivalent to the energy gained by ice.
The energy lost by water could be defined as
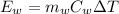
Where,
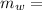 mass of water
mass of water
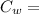 Specific Heat of Water
Specific Heat of Water
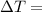 Change in Temperature
Change in Temperature
On the other hand the energy gained by ice can be defined as
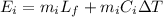
Where,
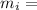 Mass of ice
Mass of ice
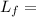 Latent heat of fusion
Latent heat of fusion
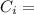 Specific heat of Ice
Specific heat of Ice
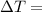 Change in Temperature
Change in Temperature
As the two energies are in balance then
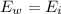
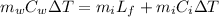
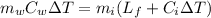
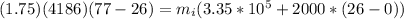
Re-arrange to find
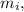
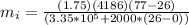
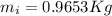
Therefore there must be 0.9653Kg of Ice