Answer: The EMF of the cell is coming when the cell having diluted concentration is getting oxidized.
Step-by-step explanation:
We are given a cell which contains two
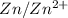 half cells. This means that the standard electrode potential of the cell will be 0.
half cells. This means that the standard electrode potential of the cell will be 0.
For a reaction to be spontaneous, the EMF of the cell must be positive. If the EMF of the cell is negative, the reaction will be non-spontaneous and will not take place.
For a reaction to be spontaneous, the diluted cell must get oxidized.
The half reaction for the given cell follows:
Oxidation half reaction:
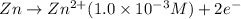
Reduction half reaction:
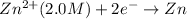
Net reaction:
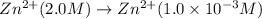
To calculate the EMF of the cell, we use Nernst equation:
![E_(cell)=E^o_(cell)-(0.0592)/(n)\log \frac{[Zn^(2+)_\text{{(diluted)}}]}{[Zn^(2+)_{\text{(concentrated)}}]}](https://img.qammunity.org/2020/formulas/chemistry/college/n65olj28i1m2zuol10v43xzwqaxrq9y8dv.png)
where,
n = number of electrons in oxidation-reduction reaction = 2
 = ?
= ?
![[Zn^(2+)_{\text{(diluted)}}]](https://img.qammunity.org/2020/formulas/chemistry/college/e5yaxfrykwtzta1kmarzjg83udicie96us.png) =
=
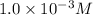
![[Zn^(2+)_{\text{(concentrated)}}]](https://img.qammunity.org/2020/formulas/chemistry/college/3l6b480d3yt2qd73zaa68avp7oqm2c40jj.png) = 2.0 M
= 2.0 M
Putting values in above equation, we get:
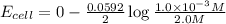
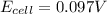
Hence, the EMF of the cell is coming when the cell having diluted concentration is getting oxidized.