Answer: The other product in the given reaction is sodium hydroxide
Step-by-step explanation:
A balanced chemical equation is defined as the equation in which total number of individual atoms on the reactant side is equal to the total number of individual atoms on product side.
When a metal reacts with water, it leads to the formation of metal hydroxide and hydrogen gas
The balanced chemical equation for the reaction of sodium metal with water follows:
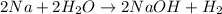
By Stoichiometry of the reaction:
2 moles of sodium metal reacts with 2 moles of water to produce 2 moles of sodium hydroxide and 1 mole of hydrogen gas
Hence, the other product in the given reaction is sodium hydroxide