Answer:
Lead will have the largest increase in temperature.
Step-by-step explanation:
Given:
The heat supplied to three equal mass blocks is same. The initial temperature of each block is 25 °C. The three blocks are of different material- iron, copper and lead.
The heat supplied to a body is directly proportional to its mass, specific heat capacity and the increase in temperature and is given as:
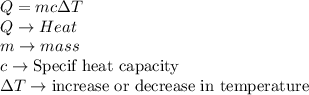
Now, for the given question, for the three blocks,
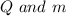 are same. Thus, specific heat capacity is inversely proportional to change in temperature.
are same. Thus, specific heat capacity is inversely proportional to change in temperature.
This means that the block that has the highest value of
 has the least increase in temperature,
has the least increase in temperature,
 and the one that has the least value of
and the one that has the least value of
 has the largest increase in temperature.
has the largest increase in temperature.
Specific heat capacity of the three blocks are:
Lead:
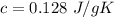
Copper:
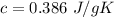
Iron:
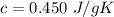
Thus, lead has the least value of
 . So, it will have the largest increase in temperature.
. So, it will have the largest increase in temperature.