Step-by-step explanation:
In case of aryl bromides, the lone pair present on bromine is involved in resonance with the benzene ring attached to it. As a result, it is less likely available for further reaction.
Therefore, the reaction will be as follows.
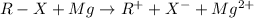
Since, there is a positive charge on R group (aryl group). Hence, it will not remain resonance stabilized and therefore, it is unstable due to which the reaction will be slow in nature.
On the other hand, there is no resonance delocalization occurring in alkyl bromide. Therefore, it will occur at a faster rate as compared to aryl bromides.