Answer: The value of
 is
is
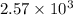 and the reaction must proceed in the forward direction.
and the reaction must proceed in the forward direction.
Step-by-step explanation:
The given chemical equation follows:
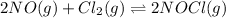
Relation of
 with
with
 is given by the formula:
is given by the formula:
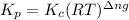
Where,
 = equilibrium constant in terms of partial pressure = ?
= equilibrium constant in terms of partial pressure = ?
 = equilibrium constant in terms of concentration =
= equilibrium constant in terms of concentration =

R = Gas constant =
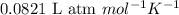
T = temperature =
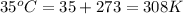
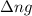 = change in number of moles of gas particles =
= change in number of moles of gas particles =
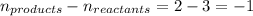
Putting values in above equation, we get:
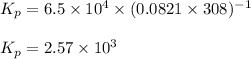
 is the constant of a certain reaction at equilibrium while
is the constant of a certain reaction at equilibrium while
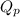 is the quotient of activities of products and reactants at any stage other than equilibrium of a reaction.
is the quotient of activities of products and reactants at any stage other than equilibrium of a reaction.
The expression of
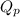 for above equation follows:
for above equation follows:
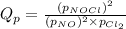
We are given:
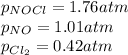
Putting values in above equation, we get:
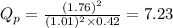
We are given:
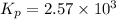
There are 3 conditions:
- When
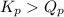 ; the reaction is product favored.
; the reaction is product favored. - When
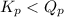 ; the reaction is reactant favored.
; the reaction is reactant favored. - When
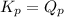 ; the reaction is in equilibrium.
; the reaction is in equilibrium.
As,
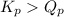 , the reaction will be favoring product side.
, the reaction will be favoring product side.
Hence, the value of
 is
is
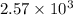 and the reaction must proceed in the forward direction.
and the reaction must proceed in the forward direction.