Answer:
The amount of work required for the heart is: 3.14 Joules
Step-by-step explanation:
We need to remember that the work done for a pump with constant pressure will be:
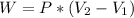 , where W is the work, P is pressure and V is volume (change of the volume). Now we need to find the pressure. Using the Pascal's law (
, where W is the work, P is pressure and V is volume (change of the volume). Now we need to find the pressure. Using the Pascal's law (
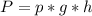 ), where P is pressure, p is density, g is the gravity and h is the height of the fluid's column and replacing the values given, we can find the pressure:
), where P is pressure, p is density, g is the gravity and h is the height of the fluid's column and replacing the values given, we can find the pressure:
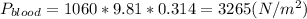 . Then using the work's equation and replacing the value obtain for the pressure and the volume we get:
. Then using the work's equation and replacing the value obtain for the pressure and the volume we get:
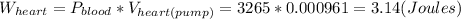 .
.