Answer:
31.3 kJ/mol is the free energy, ΔG, for the overall reaction, A⇌D.
Step-by-step explanation:
A⇌B,
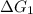 = 11.9 kJ/mol ...[1]
= 11.9 kJ/mol ...[1]
B⇌C,
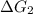 = -26.7 kJ/mol ...[2]
= -26.7 kJ/mol ...[2]
C⇌D,
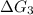 = 7.30 kJ/mol ...[3]
= 7.30 kJ/mol ...[3]
A⇌D,
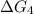 = ?...[4]
= ?...[4]
[4] = [1] - [2] - [3] (Using Hess's law)
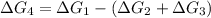
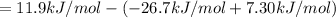
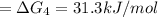
31.3 kJ/mol is the free energy, ΔG, for the overall reaction, A⇌D.