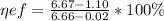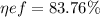We need the Carbon Iron diagram to be able to solve this exercise.
Basically we make a line that is at the temperature of 727 ° C
If we make the line in such a way that it crosses the entire plane we can notice that there is no formation of Proeutectoid ferrite and only there is eutectoid ferrite.
PART A) From that line we can find the weight percent of proeutectid cementite, at which is:
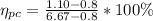

PART B) At the same way the total cementite would be
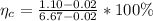
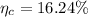
The the weight percent of total eutectoid cementite would be
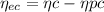
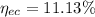
At the end the percent of eutectoid ferrite is