Answer:
a) The theoretical yield is 408.45g of
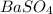
b) Percent yield =
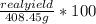
Step-by-step explanation:
1. First determine the numer of moles of
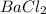 and
and
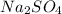 .
.
Molarity is expressed as:
M=
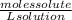
- For the
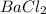
M=
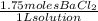
Therefore there are 1.75 moles of
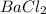
- For the
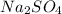
M=
![\frac{2.0moles[tex]Na_(2)SO_(4)]() }{1Lsolution}[/tex]
}{1Lsolution}[/tex]
Therefore there are 2.0 moles of
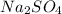
2. Write the balanced chemical equation for the synthesis of the barium white pigment,
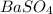 :
:
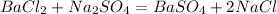
3. Determine the limiting reagent.
To determine the limiting reagent divide the number of moles by the stoichiometric coefficient of each compound:
- For the
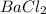 :
:
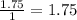
- For the
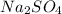 :
:
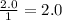
As the
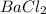 is the smalles quantity, this is the limiting reagent.
is the smalles quantity, this is the limiting reagent.
4. Calculate the mass in grams of the barium white pigment produced from the limiting reagent.
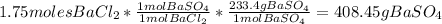
5. The percent yield for your synthesis of the barium white pigment will be calculated using the following equation:
Percent yield =
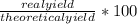
Percent yield =
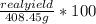
The real yield is the quantity of barium white pigment you obtained in the laboratory.