Answer:
272.43 K or -0.718°C
Step-by-step explanation:
We are given;
The initial pressure,P1 as 761 mmHg
Initial temperature, T1 as 0.00°C which is equivalent to 273.15 K
Final pressure as 759 mmHg
We are required to calculate the final temperature;
According to pressure law, the pressure of a gas and absolute temperature are directly proportional at constant volume.
That is; Pα T
Therefore, at varying pressure and temperature,
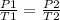
To get final temperature;
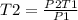
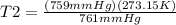
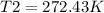
Therefore, the final temperature will be 272.43 K or -0.718°C