75 g of sodium hydroxide is present in the solution.
Step-by-step explanation :
Given :
Molarity of sodium hydroxide solution= 2.5 M
Litres of Solution = 750 mL
Molar mass of NaOH = 40 g/mol
To Find:
Grams of Sodium Hydroxide =?
Solution :
Molarity of the solution is expressed in Litres, So Converting 750.0 mL to Litres we have
750.0mL=0.750 Litres
Grams of Solution , moles=
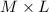
Where
M = Molarity
L = litres of solution
mol= moles of solute
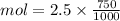
Mol = 1.875
Also the molecular weight of NaOH is 40.00 g/mol
Grams of sodium hydroxide = 1.875 mol * 40.00 g/mol = 75 g