Answer:
32 g
Explanation:
We are given that
Mass of lithium=14 g
Mass of lithium sulfide=46 g
The reaction between lithium and sulfur is given below:
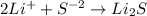
14 g 46 g
According to law of mass conservation:
Mass of reactants=Mass of products
Mass of lithium+mass of sulfur=Mass of lithium sulfide
14g+mass of sulfur=46g
Mass of sulfur=46-14=32 g
Hence, mass of sulfur=32 g