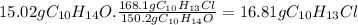Answer:
16.81 g of C₁₀H₁₃Cl
Step-by-step explanation:
When 3-phenyl-2-butanol reacts with HCl it undergoes a substitution reaction to form 2-chloro-3-phenylbutane and water.
C₁₀H₁₄O + HCl ⇄ C₁₀H₁₃Cl + H₂O
The molar mass of 3-phenyl-2-butanol is 150.2 g/mol and the molar mass of 2-chloro-3-phenylbutane is 168.1 g/mol. The molar ratio is 1:1. Then, for 15.02 g of 3-phenyl-2-butanol, the theoretical yield is: