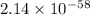Answer :
(a) The value of
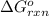 for the biological synthesis of ammonia at 298 K is
for the biological synthesis of ammonia at 298 K is
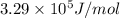
(b) The value of equilibrium constant for the biological synthesis of ammonia is
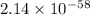
Explanation :
(a) The equation used to calculate
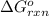 of a reaction is:
of a reaction is:
![\Delta G^o_(rxn)=\sum [n* \Delta G^o_f(product)]-\sum [n* \Delta G^o_f(reactant)]](https://img.qammunity.org/2020/formulas/chemistry/college/bh478msmvp7ei02bm3b8wrhfppzsl1vrdo.png)
The equilibrium reaction follows:
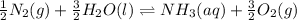
The equation for the
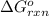 of the above reaction is:
of the above reaction is:
![\Delta G^o_(rxn)=[(n_((NH_3))* \Delta G^o_f_((NH_3)))+(n_((O_2))* \Delta G^o_f_((O_2)))]-[(n_((N_2))* \Delta G^o_f_((N_2)))+(n_((H_2O))* \Delta G^o_f_((H_2O)))]](https://img.qammunity.org/2020/formulas/chemistry/college/6eubxun220o6pqlwrba1eqestw9ffx1z8h.png)
We are given:
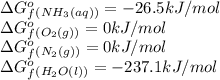
Putting values in above equation, we get:
![\Delta G^o_(rxn)=[(1* -26.5)+((3)/(2)* -0)]-[((1)/(2)* 0)+((3)/(2)* -237.1)]=329.15kJ/mol=3.29* 10^5J/mol](https://img.qammunity.org/2020/formulas/chemistry/college/lnp6ihbopf5xr8jjb8hfv8kjlbef9bwi3z.png)
Thus, the value of
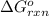 for the biological synthesis of ammonia at 298 K is
for the biological synthesis of ammonia at 298 K is
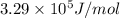
(b) Now we have to calculate the value of equilibrium constant.
Formula used :
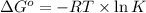
where,
R = universal gas constant = 8.314 J/K/mole
T = temperature = 298 K
K = equilibrium constant = ?
Now put all the given values in this formula, we get the value of K.
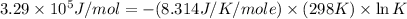
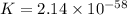
Therefore, the value of equilibrium constant for the biological synthesis of ammonia is