Step-by-step explanation:
As a nitrogen molecule (
 ) contains a triple bond between the two nitrogen atoms. Therefore, there is more overlapping taking place as there are two pi bonds and one sigma bond present within a
) contains a triple bond between the two nitrogen atoms. Therefore, there is more overlapping taking place as there are two pi bonds and one sigma bond present within a
 molecule.
molecule.
Hence, in order to break a triple bond high amount of energy is required.
On the other hand, in a
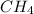 all the hydrogen atoms are bonded by a single bond with the hydrogen atom. Hence, there are 4 sigma bonds present in a
all the hydrogen atoms are bonded by a single bond with the hydrogen atom. Hence, there are 4 sigma bonds present in a
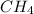 molecule.
molecule.
Also, a pi bond is much stronger than a sigma bond.
That is why,
 molecule requires more energy to break the bond.
molecule requires more energy to break the bond.