Step-by-step explanation:
Relation between pressure, latent heat of fusion, and change in volume is as follows.
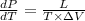
Also,
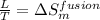
where,
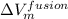 is the difference in specific volumes.
is the difference in specific volumes.
Hence,
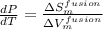
As,
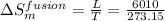 = 22.0 J/mol K
= 22.0 J/mol K
And,
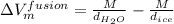 ...... (1)
...... (1)
where,
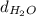 = density of water
= density of water
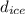 = density of ice
= density of ice
M = molar mass of water =
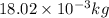
Therefore, using formula in equation (1) we will calculate the volume of fusion as follows.
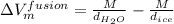
=

=
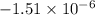
Therefore, calculate the required pressure as follows.
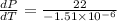
=
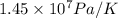
or, = 145 bar/K
Hence, for change of 1 degree pressure the decrease is 145 bar and for 4.7 degree change dP =
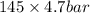
= 681.5 bar
Thus, we can conclude that pressure should be increased by 681.5 bar to cause 4.7 degree change in melting point.