Answer:
544.34 mL
Step-by-step explanation:
- Combined gas law is a law that combines both Boyle's law and Charles's law.
- According to the combined gas law the volume of a fixed mas of a gas is directly proportional to absolute temperature but inversely proportional to pressure.
That is;
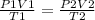
in our case we are given;
Initial volume, V1 = 1025 mL
Initial temperature, T1 = 75 °C (But K = °C + 273.5)
= 348.5 K
Initial pressure, P1 = 0.75 atm
New temperature, T2 = 308.15 K (35°C +273.15)
New pressure , P2 = 1.25 atm
We are required to calculate the new volume, V2
Replacing the known variables in the equation of combined gas law we can find the new volume;
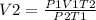
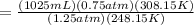
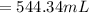
Therefore, the new volume is 544.34 mL