Answer:
7.182K
Step-by-step explanation:
From the question we are given;
- Initial temperature, T1 = 275 K
- Final temperature, T2 = 395 K
- Initial volume, V1 = 5 L
We are required to calculate the final volume, V2
- Charles's law is the law that relates the volume of a gas and its temperature.
- It states that the volume of a fixed mass of a gas and its absolute temperature are directly proportional at a constant pressure.
- Therefore;
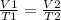
To calculate, V2 we rearrange the formula;
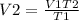
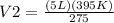
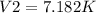
Therefore, the ending volume will be 7.182K