Answer:
F = 2.3 x 10⁻⁸ N
Step-by-step explanation:
Given,
The mean distance between the electron and the nucleus, x = 1 x 10⁻¹⁰ m
The number of protons present in the nucleus of the hydrogen atom is 1.
The charge of the proton and electron, q = 1.602 x 10⁻¹⁹ C
Since they have opposite charges, the force between them is attractive,
The coulomb law of force between two charges is given by the formula,
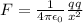
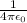 = 9 x 10⁹ Nm²C⁻²
= 9 x 10⁹ Nm²C⁻²
Substituting the values in the above equation
F = 9 x 10⁹ X (1.602 x 10⁻¹⁹)² / (1 x 10⁻¹⁰)²
= 2.31 x 10⁻⁸ N
Hence, the force between the nucleus and electron of hydrogen atom is, F = 2.3 x 10⁻⁸ N