Answer:
≈ 1833
Explanation:
To find ratio of proton mass to electron mass, we have to divide.
The numbers are given in scientific notation.
Let a number be
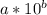 and another be
and another be
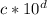 , when we divide, we will follow the rule shown below:
, when we divide, we will follow the rule shown below:
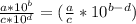
Now, we use the information given to find the ratio:
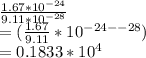
This means we can find the number by taking 4 decimal places to the right, so that would becomes:
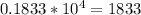
The approximate ratio is 1833 [mass of proton is around 1833 times heavier than mass of electron]