Answer:
620.71 L the final volume of the balloon.
Step-by-step explanation:
Initial volume of the gas in the balloon=
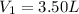
Initial pressure of the gas in the balloon=
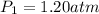
Initial temperature of the gas in the balloon=
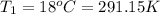
Moles of gases = n
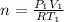 ...[1]
...[1]
Final volume of the gas in the balloon =
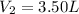
Final pressure of the gas in the balloon =
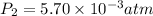
Final temperature of the gas in the balloon =
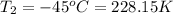
Moles of gases = n
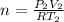 ...[2]
...[2]
[1] = [2]
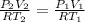
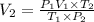
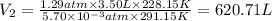
620.71 L the final volume of the balloon.