Answer :
(1) The entropy will also decreases.
(2) The entropy will also decreases.
(3) The entropy will also increases.
(4) The entropy will also increases.
(5) The entropy will also decreases.
Explanation :
Entropy : It is defined as the measurement of randomness or disorderedness in a system.
The order of entropy will be,
As we are moving from solid state to liquid state to gaseous state, the entropy will be increases due to the increase in the disorderedness.
As we are moving from gaseous state to liquid state to solid state, the entropy will be decreases due to the decrease in the disorderedness.
(1)
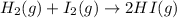
In this reaction, the randomness of reactant molecules are more and as we move towards the formation of product the randomness become less that means the degree of disorderedness decreases. So, the entropy will also decreases.
(2)
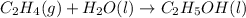
In this reaction, the randomness of reactant molecules are more (gas+liquid) and as we move towards the formation of product the randomness become less (liquid) that means the degree of disorderedness decreases. So, the entropy will also decreases.
(3)
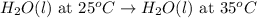
In this reaction, as we move towards the formation of product the disorderedness will increases due to increase in temperature. So, the entropy will also increases.
(4)
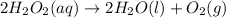
In this reaction, the randomness of reactant molecules are less and as we move towards the formation of product the randomness become more that means the degree of disorderedness increase. So, the entropy will also increases.
(5)
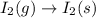
In this reaction, the phase changes from gaseous state to solid state that means the degree of disorderedness decreases. So, the entropy will also decreases.