Carbon 14 change back into the stable nuclei Nitrogen by emitting electron and electron antineutron.
Explanation:-
Carbon 14 is one of the radioactive isotope elements that contains 6 protons and 8 neutron elements.
A general chemical reaction of Carbon 14 is given below.
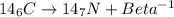
This above reaction can be categorized under Nuclear Reaction. When atomic number 6 (Carbon) was irradiated, Beta ray emission happen and new stable nuclei with atomic number 7 (Nitrogen) formed without change in atomic mass of any nuclei. As well as some amount of energy also emitted through this reaction.