Answer:
the difference in parent nuclei and daughter nuclei is that daughter nuclei is having smaller mass number and by 4 and smaller atomic number by 2
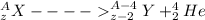
Step-by-step explanation:
Alpha nucleus is the helium nuclei which is having 2 protons and 2 neutrons
So we will have
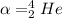
now we know that in all radioactive decay mass number and atomic number will remain constant on product and reactant side
So we have
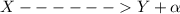
now by mass number conservation and atomic number conservation we have
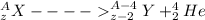
so the difference in parent nuclei and daughter nuclei is that daughter nuclei is having smaller mass number and by 4 and smaller atomic number by 2