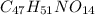Answer:
The empirical formula is =
Step-by-step explanation:
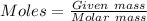
% of C = 66.11
Molar mass of C = 12.0107 g/mol
% moles of C = 66.11 / 12.0107 = 5.5043
% of H = 6.02
Molar mass of H = 1.00784 g/mol
% moles of H = 6.02 / 1.00784= 5.973
% of N = 1.64
Molar mass of N = 14.0067 g/mol
% moles of N = 1.64 / 14.0067= 0.1171
Given that the Paclitaxel contains C, H, N and O. So,
% of O = 100% - % of C - % of H - % of N = 100 - 66.11 - 6.02 - 1.64 = 26.23 %
Molar mass of O = 15.999 g/mol
% moles of O = 26.23 / 15.999 = 1.6395
Taking the simplest ratio for C, H, N and O as:
5.5043 : 5.973 : 0.1171 : 1.6395
= 47 : 51 : 1 : 14
The empirical formula is =