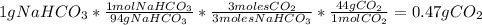Answer:
0.47g of

Step-by-step explanation:
1. Balanced equation:
3NaHCO₃(aq) + H₃C₆H₅O₇(aq) --------> 3CO₂(g) + 3H₂O(l) + Na₃C₆H₅O₇(aq)
2. Find the limiting reagent between the reactants:
-For the
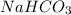 :
:
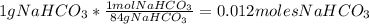
Divide the number of moles between the stoichiometric coefficient of the
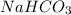 :
:
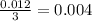
-For the
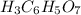 :
:
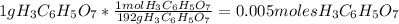
Divide the number of moles between the stoichiometric coefficient of the
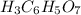 :
:
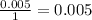
The smallest number is the 0.004 therefore, the limiting reagent is the sodium bicarbonate.
3. Calculate the grams of carbon dioxide: