Answer:
The enthalpy of reaction when chlorine reacts with ozone is -162.5 kJ/mol.
Step-by-step explanation:
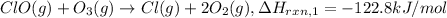 ..[1]
..[1]
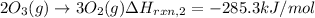 ..[2]
..[2]
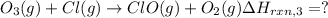 ...[3]
...[3]
Applying Hess's law:
Hess’s law of constant heat summation states that the amount of heat absorbed or evolved in a given chemical equation remains the same whether the process occurs in one step or several steps.
[2] - [1] = [3]
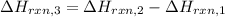
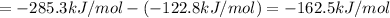
The enthalpy of reaction when chlorine reacts with ozone is -162.5 kJ/mol.