Answer:
a) m = 90.39 10-3 kg , b) P2 = 336 kPa , c) Q = 0.1465 kJ and d)
friction force
Step-by-step explanation:
The air behaves in the first approximation as an ideal gas so we will use the ideal gas equation
P V = n R T
Where P, V and T are the pressure, volume and absolute temperature (Kelvin), n is the number of moles and R is the gas constant with a value of 8.314 J / mol K
a) Let's calculate the number of moles of gas
T = 273 +25 = 298K
P = 100 103 + 210 103 = 310 103 Pa
n = PV / RT
n = 310 103 0.025 / (8.314 298)
n = 3.12 mol
The air is mainly composed of two gases 78% nitrogen and oxygen 21, the Tor 1% are several gases (CO2, water vapor, etc.), we will neglect this 1%.
Atomic weights oxygen = 16 g / mol
Atomic weight Nitrogen = 14 g / mol
The molecule of each gas is formed by two atoms, so the molecular weights (PM) are
Oxygen PM = 2 16 = 32 g / mol
PM nitrogen = 2 14 = 28 g / mol
Molecular weight of air is
Pm air = 32 0.21 + 28 0.78 = 28.56 g / mol
The molecular weight value of the tables is 28.97 g/mol, for the calculation we will use this value
Now let's use the definition of mol
n = m / PM
m = PM n
m = 28.97 3.12
m = 90.39 g
m = 90.39 10-3 kg
b) To find the final pressure, write the equation of the ideal gases for the two points
P1 V = n R T1
P2 V = n R T2
Resolve and calculators
T1 = 298K
T2 = 273 +50 = 323K
P1 / P2 = T1 / T2
P2 = p1 T2 / T1
P2 = 310 103 323/298
P2 = 336 103 Pa
P2 = 336 kPa
c) The heat transferred
Q = m
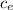 ΔT = m
ΔT = m
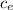 (
(
 -T₀)
-T₀)
We need to find the specific heat of the air, let's use redefined specific heat that is derived from the energy with respect to the constant volume temperature. Energy
U = 5/2 NKT = 5/2 nRT
Where 5 comes from the fact that the molecule is diatomic, for monatomic molecular 3
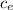 = (dU/dT) v = 5/2 nR
= (dU/dT) v = 5/2 nR
We replace we calculate
Q = m 5/2 nR (
 -T₀)
-T₀)
Q = 5/2 90.39 3.12 8.314 (323-298)
Q = 146.5 J
Q = 0.1465 kJ
d) the source of this heat is between the tires and the pavement,
friction force