Answer:
λ=1.7pm
This electron would not be useful in a Davisson-Germer type scattering experiment.
θ=0.45⁰
Step-by-step explanation:
a) We need to use the relativistic for of the kinetic energy:
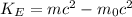 . (1)
. (1)
Here m₀ is the mass rest and c the speed of light in vacuum.
We can write this equation in terms of the linear momentum (p) using this expression:
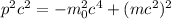 (2)
(2)
if we solve this equation for p and put into the first equation we will have the KE in terms of p.
From (2) we have:
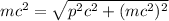 (3)
(3)
Let's substitute mc² in (1).
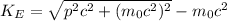 (4)
(4)
Now, let's solve this for p:
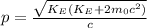
And finally, using the De Broglie wavelength equation
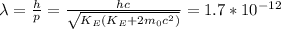 .
.
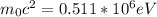

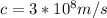
b) To determine the angle of the first order diffraction, we use the Bragg equation:
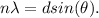
Now , in the Davisson and Germer, they use a nickel target, so the inter atomic distance for this particular element is around 0.215 nm. We take n=1 for the first order
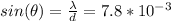 and
and
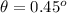 .
.
If we see, this angle is to small to implement the experiment, so it would not be useful in a Davisson-Germer type scattering experiment.