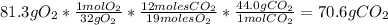Answer:
The maximum mass of carbon dioxide that could be produced by the chemical reaction is 70.6gCO_{2}
Step-by-step explanation:
1. Write down the balanced chemical reaction:
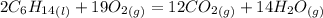
2. Find the limiting reagent:
- First calculate the number of moles of hexane and oxygen with the mass given by the problem.
For the hexane:
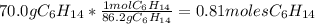
For the oxygen:
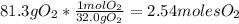
- Then divide the number of moles between the stoichiometric coefficient:
For the hexane:
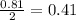
For the oxygen:
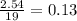
- As the fraction for the oxygen is the smallest, the oxygen is the limiting reagent.
3. Calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction:
The calculations must be done with the limiting reagent, that is the oxygen.