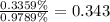Answer:
The ratio of percent dissociation of the 1.59 M solution to the 0.186 M solution: 0.343 : 1.
Step-by-step explanation:
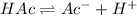
Initially c
At equilibrium c-cα cα cα
Dissociation constant of an acetic acid:
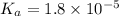
Degree of dissociation = α
Dissociation constant of an acid is given by:
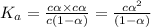
1) Concentration of acid = c = [HAc] = 1.59 M
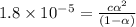
Degree of dissociation = α
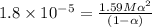
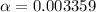
Percentage of dissociation = 0.3359%
2) Concentration of acid = c' = [HAc] = 0.186 M
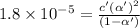
Degree of dissociation = α'
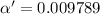
Percentage of dissociation = 0.9789%
The ratio of percent dissociation of the 1.59 M solution to the 0.186 M solution: