Answer:
The % of of acid in solution = 63.88 ml
Explanation:
The solution of acid and water = 351 milliliters
The % of of acid in solution = 18.2% of 351 ml
Or, The % of of acid in solution =
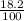 × 351 ml
× 351 ml
Or, The % of of acid in solution = 63.882 ml
Hence ,The % of of acid in solution = 63.88 ml Answer