Answer:
Mass/mass percent accounts for a relationship between the solute's mass that is contained into a particular solution's mass as well
Step-by-step explanation:
Hello,
Mass/mass percent accounts for a relationship between the solute's mass that is contained into a particular solution's mass as well. Such relationship is numerically defined via:
%
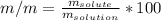 %
%
Whereas
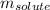 is the given solute's mass and
is the given solute's mass and
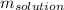 is the computed or given solution's mass (is the addition between the solute's and solvent's mass).
is the computed or given solution's mass (is the addition between the solute's and solvent's mass).
Best regards.