Answer:
%
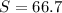 %
%
Step-by-step explanation:
Hello,
Sulfur monoxide, SO, has a molecular mass of 48g/mol (32g/mol for S and 16g/mol for O), thus, the present sulfur percent is given by the following equation as long as there's only one sulfur in the formula:
%
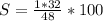 %
%
%
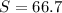 %
%
Best regards.