Answer: The molar enthalpy change is 73.04 kJ/mol
Step-by-step explanation:

moles of HCl=
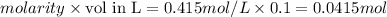
As NaOH is in excess 0.0415 moles of HCl reacts with 0.0415 moles of NaOH.
volume of water = 100.0 ml + 50.0 ml = 150.0 ml
density of water = 1.0 g/ml
mass of water =
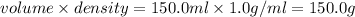
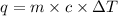
q = heat released
m = mass = 150.0 g
c = specific heat =
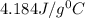
 = change in temperature =
= change in temperature =
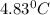
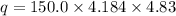
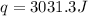
Thus 0.0415 mol of HCl produces heat = 3031.3 J
1 mol of HCL produces heat =
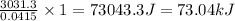
Thus molar enthalpy change is 73.04 kJ/mol